A Center of Excellence for Poultry Research
Agricultural Research Service (ARS) scientists working in Athens, Georgia, are world renowned for their expertise not only in identifying the latest bird flu threats but also in countering them through basic and applied research. Ongoing studies include the host responses of wild birds and poultry, vaccine efficacy and improvement, virus epidemiology, food safety, and diagnostic testing.
Led by David Suarez, the ARS Exotic and Emerging Avian Viral Diseases Research Unit in Athens is among the first to investigate new reports of avian influenza viruses and assess their threat to poultry and, in some cases, their impact on people (in conjunction with state and federal public-health agencies). Suarez’s team analyzes the genetic sequences of new avian influenza viruses and performs database searches to determine whether they match previously described viruses. Of particular concern are viruses highly pathogenic to chickens, turkeys, domesticated ducks, and other poultry species.
The U.S. National Poultry Research Center, where Suarez’s unit is located, houses a Biosafety Level 3 facility that enables the team to study live specimens of avian influenza viruses. “That’s when we can really do indepth research,” says Suarez. The specimens come from all over the world, but the primary collaborator is the U.S. Department of Agriculture’s National Veterinary Services Laboratories in Ames, Iowa.
A first order of business is using a reference epidemic strain to conduct host-response studies in research flocks of poultry. This involves exposing the birds to the strain to gauge their susceptibility to it and learn how it replicates, causes disease, and spreads. The work establishes baseline information about the unique aspects of each virus, which can assist with surveillance and flock-monitoring programs, devising control measures, guiding regulatory action, and other endeavors.
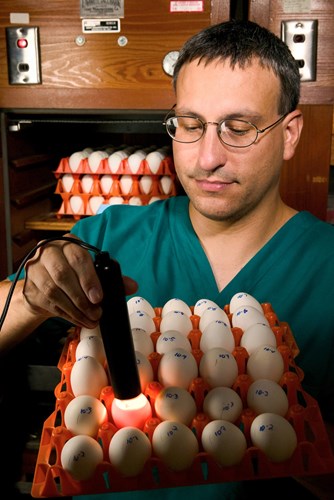 ARS veterinary medical officer David Suarez shines light through chicken eggs to look for signs of life. Embryo death may indicate viral infection. (Stephen Ausmus, D240-8) |
Given the global nature of the avian influenza threat, sharing information is another facet of the team’s efforts. In 2013, for example, through investigations into a virus type (H7N9) circulating in China, Suarez’s group showed that the virus replicated in the respiratory tract of poultry rather than the intestinal tract, where public health officials had mainly collected samples for testing. “Our findings suggested that, in the field, the birds’ respiratory tract was the best place to look for it,” says Suarez.
In another instance, investigations of the H5N2 subtype in the United States during the 2014-2015 outbreak showed that the virus had a far longer incubation time in turkeys than expected—5 to 6 days versus the 2 to 3 days observed with other subtypes. Suarez says that a longer incubation period raised the risk of producers transporting or marketing turkeys that appeared healthy but were actually infected and thus able to spread the virus to neighboring flocks. The outbreak of H5N2, together with two closely related subtypes, ultimately led to the deaths or culling of nearly 50 million U.S. chickens and turkeys.
When new outbreaks occur or new strains are found (such as the highly pathogenic H7 subtype confirmed this past March in a Tennessee flock of 73,500 chickens), Suarez’s team tests the effectiveness of existing detection methods and approved vaccines—and improves them if necessary. For example, they’ll compare the genetics of the viruses used to make existing vaccines with the genetics of newly reported strains. Generally, the closer the match, the better the vaccine can protect birds from a new threat.
When a vaccine performs poorly in trials, the researchers report their results to poultry producers, vaccine makers, and organizations charged with stockpiling the vaccines. During the 2014-2015 outbreak, researchers found vaccines available at the beginning of the outbreak were inadequate in preventing the shed and spread of avian influenza viruses. So, using an approach called “reverse genetics,” they created a new vaccine containing a weakened H5 hemagglutinin protein. They then worked with a commercial partner to develop, test, and attain regulatory approval for the vaccine.
Approaches such as reverse genetics enable the researchers to delve into the complexities of the virus’s disease-causing machinery and its ability to mutate and “pilfer” genetic material from its hosts, potentially adding to its virulence. Of particular interest is assessing the potential for low-pathogenic avian influenza subtypes to become highly pathogenic in poultry and, in rare instances, infect humans, which is a public-health concern.
Since the mid-1990s, the researchers have led in the development of new methods to detect and characterize avian influenza viruses. Some tests have become frontline tools used worldwide, like the Spackman RRT-PCR test, named after Erica Spackman, the ARS microbiologist who helped develop it. However, rather than rest on past successes, Spackman, Suarez, and colleagues are pushing to make avian influenza testing even faster, more sensitive, and easier to use. For example, the team is exploring ways to create vaccines that can be mass-administered to large flocks, such as via sprays.
In collaboration with its state, federal, and international partners, the ARS facilities in Athens will continue serving as a hub of research excellence in responding to and anticipating emerging avian influenza threats. “When you have an outbreak, you need the research done immediately, and we have the capability, knowledge, and expertise to do that,” says Suarez.—By Jan Suszkiw, ARS Office of Communications.
----------------------------------------------------------------------------------------------------------------------------------
 When a new avian influenza virus is detected, ARS scientists check to see whether existing vaccines can protect birds. (Stephen Ausmus, K10004-11) |
BONUS CONTENT
Attention Bird Flu: Keep Out!
Research is only one part of avian influenza prevention and management. Commercial poultry producers and backyard hobbyists alike can play a part, starting with biosecurity.
The term refers to measures that anyone who owns or works with captive poultry can take to keep avian influenza away. For commercial producers, these protective practices cover four main groups: premises, personnel, equipment, and vehicles.
As described by the U.S. Department of Agriculture’s Animal and Plant Health Inspection Service’s “Defend the Flock” and “Biosecurity for Birds” campaigns, safeguarding practices for premises include using signs, locking poultry house entrances, and securing feed bins. Personnel measures include providing biosecurity training and counseling to employees. Equipment- and vehicle-related measures include using footbaths and sanitizing stations at entrances and cleaning and disinfecting vehicles that stop at locations where other birds are present.
Backyard biosecurity focuses on six steps:
• Restricting access to areas where birds are kept;
• Cleaning and disinfecting hands, shoes, equipment, cages, and the like;
• Restocking the flock from reputable sources and keeping newly acquired birds separated for 2 to 4 weeks.
• Avoiding sharing birds or equipment with other people—or being sure to disinfect any borrowed tools;
• Keeping a sharp eye out for warning signs of avian influenza; and
• Reporting sick birds promptly to the local or state veterinarian, extension agent, or animal health diagnostic laboratory.
Learn more about biosecurity for birds here and here.
Key Facts
- ARS scientists in Athens, Georgia, are world renowned in studying bird diseases.
- Some avian influenza viruses can kill poultry and infect humans.
- Avian influenza is a global threat requiring international collaboration.
- An ARS scientist developed a test used worldwide to detect avian influenza viruses.
Full Story