Roundworms (and Their Bacterial Buddies) Rub Out Pests
Roundworms, microscopic wormlike organisms also known as “nematodes,” can be friend, foe, or something in between. Some species are parasitic, dependent on hosts—plant, animal, insect, or human—to survive. Others are stealthy predators, prowling soils and other hunting grounds for bacteria, fungi, and other microbial prey—or sometimes ambushing them.
Lynn Carta and colleagues at the Agricultural Research Service’s Nematology Laboratory in Beltsville, Maryland, are among the world’s foremost authorities on nematodes and have developed sophisticated tools and techniques for studying their morphological, biochemical, and genetic features in unprecedented detail.
Not surprisingly, the lab’s services are in high demand, especially from regulatory agencies tasked with safeguarding U.S. agriculture from the entry of exotic pathogens and pests, such as the pale cyst nematode, Globodera pallida, which damages potato, tomato, and other crops.
The ARS lab’s expertise is also critical to research aimed at harnessing beneficial species of insect-killing (entomopathogenic) nematodes as biocontrol agents, which can be commercially formulated as an alternative to synthetic pesticides.
|
|
Casting About for New Talent
Lately, Carta has focused attention on identifying and describing nematodes that have potential as biological controls for Formosan subterranean termites, a nonnative species whose appetite for cellulose—whether it be the heartwood of trees or the wooden support beams of buildings—causes an estimated $1 billion annually in damages, repairs, and controls.
Since 1999, Carta has determined the identities of seven nematode species isolated from the bodies of Formosan termites by Ashok Raina, a retired entomologist formerly with ARS’s Southern Regional Research Center in New Orleans, Louisiana. Other specimens Carta has identified were collected from more exotic locales, including rural sites in Uzbekistan, where collaborators found species of Pelodera nematodes in the heads, abdomens, and legs of dead or sick Turkestan termites. The team reported its findings in the December 2010 issue of the International Journal of Nematology.
|
|
“Common entomopathogenic nematodes don’t kill invasive Formosan termites efficiently,” notes Carta. Thus, more virulent species are sought, and so are symbiotic microorganisms, such as bacteria, that can assist in killing the termites or other insect hosts. The Pelodera nematodes are of interest “because of their association with significant sickness and mortality in termites in Uzbekistan,” she says.
A Poikilolaimus nematode species that Raina collected in Louisiana is also of interest. His early experiments showed that this nematode species invades the heads of Formosan termites and that a bacterial accomplice probably sickened the insects in the field. The bacteria, which were identified by ARS microbiologist Phyllis Martin, are known to excrete trace amounts of cyanide. But whether this played a role in the termite’s deaths has yet to be determined. “We did not identify the bacterium from the termite itself, but only found it with the nematode,” says Carta.
Still, the bacterial association raises an interesting prospect: using nematodes as vectors of insect pathogens rather than as primary biocontrol agents—the traditional approach. Applied to the soil, for example, the nematodes would find, penetrate, and then infest their targeted insect hosts with lethal doses of entomopathogenic bacteria.
|
|
Setting the Stage
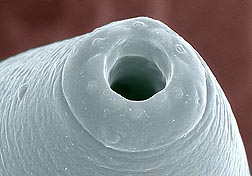
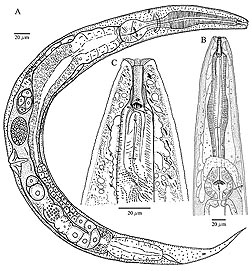
That such approaches can even be considered is a credit to the wealth of information resulting from studies by Carta and colleagues. This includes meticulous drawings that identify parts of nematodes—including their mouth and tail features—determinations of their host range and nature of their associations, geographic distribution, taxonomic groupings, genetic sequences, and other data.
It also helps to have high-tech tools handy, including a scanning electron microscope (SEM), which uses special sensors and an electron beam to produce images of specimens that far surpass what can be achieved with standard light microscopes. Eric Erbe and William Wergin, both retired and formerly with ARS’s Electron and Confocal Microscopy Unit (ECMU) in Beltsville, Maryland, are among those Carta credits with assisting in her investigations. She also credits Gary Bauchan, who now directs research at ECMU.
Carta’s recent SEM-aided collaborations include working with a team of researchers led by Cetin Yuceer from Mississippi State University and including scientists from the University of Chicago, Davidson College, Hendrix College, and the USDA Forest Service to identify a new nematode species that’s associated with the southern pine beetle, Dendroctonus frontalis.
The 1/8-inch-long beetle is a pest of coniferous forests mainly in the southeastern United States. Outbreaks are sporadic, but can be costly. For example, an outbreak from 1992 to 1993 on nearly 3,000 acres in Maryland destroyed 20 million board feet of loblolly pine, according to the state’s Department of Natural Resources.
The researchers’ studies suggest the nematode is a new species of Parasitorhabditis—similar to those found infesting beetles in parts of Texas, New York, and Germany. Besides its taxonomic significance, the nematode’s discovery may shed further light on the pest’s ecological role and success, as well as expose vulnerabilities that could be exploited.
Oh, What a Tangled Web...
Carta’s sleuthing has also helped finger a nematode-microbe duo in the deaths of pet tarantulas. The nematode, a Panagrellus species, apparently harms the spider only in captivity. The nematode is suspected of being present in some insects fed to tarantulas, including mealworms, the larval stage of a bark beetle. Once inside the tarantula’s mouth, the nematode wriggles into the spider’s head, feeding on its brain and eventually killing it. Carta believes a yeast is also involved. She is intrigued by the possibility, because it would reveal a new ecological association that could yield novel approaches to pest control.
On another front, nematology research like Carta’s may have direct bearing on the well-being of soils and plants. Applying commercially formulated bacteria to kill root-damaging nematodes or fungi, for example, can also harm nontarget species that help with nutrient recycling. Conversely, species of bacteria-feeding nematodes that occur in soils may prey on commercially formulated bacteria that have been applied, possibly reducing their biocontrol effectiveness.
The subterranean world in which nematodes operate is complex and interwoven. Knowledge gained from studies by Carta and others is improving our understanding—as well as use, management, and appreciation of—these ubiquitous organisms.—By Jan Suszkiw, Agricultural Research Service Information Staff.
This research is part of Plant Diseases, an ARS national program (#303) described at www.nps.ars.usda.gov.
Lynn Carta is with the USDA-ARS Nematology Laboratory, 10300 Baltimore Ave., Bldg. 010A, Room 110, Beltsville, MD 20705-2350; (301) 504-8787.
"Roundworms (and Their Bacterial Buddies) Rub Out Pests" was published in the August 2012 issue of Agricultural Research magazine.