IPM Targets Grasshoppers
 In large numbers, grasshoppers can strip every leaf off rangeland grasses such as this crested wheatgrass. (K7055-1) |
You could be in California, Colorado, or Nebraska.
As you step onto a grassy field, the air vibrates with sound—almost as though you'd walked into a rattlesnake pit. With each step, dozens of grasshoppers smack the grass loudly as they hop away.
Huge swarms of grasshoppers periodically sweep across western range and cropland, devouring everything in their path. Until pesticides were developed, farmers and ranchers were virtually helpless against them.
Most recently, from 1984 to 1987, grasshoppers infested 55 million acres in 17 western states. That's an area about the size of Kansas. To save forage and crops, the only option was to spray the rangeland with pesticides. Nearly 14 million acres were treated with malathion and carbaryl.
The next outbreak could come as soon as next summer.
But now, thanks to a 7-year, USDA-led project on grasshopper IPM (integrated pest management), land managers have an array of tools to help them predict and manage grasshopper outbreaks.
The toolkit holds environmentally sensitive control options, sophisticated technologies for tracking the number and life cycles of the insects, and a user-friendly computer program to guide management decisions. Research and other products are described in a Grasshopper IPM User Handbook. (See box below).
Unlike Russian wheat aphids, gypsy moths, or other exotic pests, grasshoppers are native to this country. There are hundreds of species of grasshoppers on western rangeland. About a dozen to a dozen and a half of these cause economic damage, with 5 to 7 species considered the most destructive.
Localized outbreaks—still covering thousands of acres—occur almost every year. But when environmental conditions are right, a few of these species reproduce in astounding numbers. Trillions of their offspring can spread across millions of acres in the West.
In large numbers, grasshoppers can strip every leaf off rangeland grasses. For livestock producers, that means no food for cattle and sheep.
Oregon rancher George Simmons has faced that problem the last 4 years.
 In pursuit of low-dose chemical treatments, entomologist Jerry Onsager holds a single gram of insecticide and the 12 ounces of solvent required to cover one full acre. (K7055-3) |
"I had one field of 240 acres that the grasshoppers wiped out in a week," he says.
Simmons manages 700 cows and 200 yearlings on 3,600 acres northwest of Klamath Falls, Oregon. He and five neighboring ranchers graze cattle on the 15,000 acres of private bluegrass pasture near a wildlife sanctuary.
To protect the grass for Simmons and others, USDA's Animal and Plant Health Inspection Service/Plant Protection and Quarantine sprayed 11,200 acres with malathion in July 1993. APHIS/PPQ has primary responsibility for coordinating large-scale pest control across federal, state, and private lands. Cooperators share control costs.
Ten days later, the voracious grasshoppers were back.
"The private land is next to the almost 40,000-acre Klamath Forest National Wildlife Refuge," explains Gary G. Smith, APHIS Plant Health Director for Oregon.
"Because it is difficult to avoid wetlands and other sensitive areas when spraying, we did not treat the refuge. Unfortunately, grasshopper populations there were also high. Those insects reinfested the private pastures." says Smith.
That year, Simmons' ranch spent $9,000 for forage to replace the grass lost to the hoppers. Still, the calves had to be sold early at a lower weight, for a loss of about $40 per head.
Solutions: a Special Team of Experts
 Close-up shows a grasshopper under attack by Beauvaria, one of only two biological control agents registered for use on grasshoppers. (K7055-4) |
The group came from the APHIS-led Grasshopper IPM project. The project ran from 1987 to 1994 and included a 2-year technology transfer plan. It brought together researchers, land managers, and ranchers from federal, state, and private sectors in the affected states.
"Fortunately, clear-winged grasshoppers (Camnula pellucida) gather to lay their eggs in a few small areas," says Jerome A. Onsager, who is with USDA's Agricultural Research Service. "By focusing on the egg beds, we could dramatically reduce the infestation."
An entomologist, Onsager works at the ARS Rangeland Insect Laboratory in Bozeman, Montana.
In 1995, a lure made of wheat bran laced with a very small amount of the chemical carbaryl was spread by vehicle over egg beds, to attract and kill nymphs hatching at and near the refuge. A 2,000-acre hot spot on private land was aerially treated with malathion.
"These tactics kept the grasshoppers in check and reduced our overall chemical use by 95 percent," says Smith.
Onsager, with APHIS entomologist R. Nelson Foster in Phoenix, Arizona, originally demonstrated the effectiveness of carbaryl bait and developed low-dose formulations in the 1970's. The two also served on the team that implemented the Grasshopper IPM project. The team recently received top honors from the Secretary of Agriculture.
As chemicals are still an important part of integrated pest management, scientists with the grasshopper IPM project continue to boost chemical effectiveness while lowering the amount used. On the horizon are two new chemicals: one that regulates hopper growth and another that requires only 1/270th the concentration of malathion per acre.
Going Beyond Chemicals
Scientists are also developing varied alternative control techniques. At the Klamath site, these include infecting grasshoppers with natural pathogens and attracting birds like kestrels. Also known as sparrow hawks, these reddish-brown falcons eat insects, small rodents, and lizards.
 To gauge population density, technician Jerry Mussgung (left) and insect pathologist Doug Streett set up 10-meter-square tent enclosures. (K7055-5) |
The IPM researchers continue to test Nosema locustae, a microscopic protozoan, and the fungus Beauveria bassiana—the only two biological control agents registered for grasshoppers by the U.S. Environmental Protection Agency. The researchers have also discovered at least 20 new potential biocontrol agents.
ARS entomologist Douglas A. Streett found one of the most promising: a yet-to-be-named protozoan that infects the midgut of clear-winged grasshoppers and is passed along in the feces.
"Our early tests indicate that this pathogen could be very deadly to these grasshoppers," says Streett. "Grasshoppers don't simply eat grass. They are omnivores, and many eat feces and even each other."
Street, who worked with Montana State University entomologist Kevin O'Neill, now seeks to identify this new species of protozoan and describe characteristics like its size, physical structure, and life stages. Helping with the task are ARS microbiologist Elaine A. Oma and visiting entomologist Li Ying Wang, from Beijing Agricultural University.
The minute organisms ordinarily infect grasshoppers at low levels. By blanketing an area with contaminated feces, Streett hopes to speed up the spread of the protozoa and increase grasshopper mortality.
"Although such biological control agents aren't likely to replace pesticides, they provide an alternative for areas where chemicals aren't the preferred option," says Streett.
Cultural controls offer another option. For instance, Onsager is evaluating several grazing strategies for their ability to reduce grasshopper breeding areas.
"The worst pest species need bare, dry areas in which to bask or lay their eggs," he says. "If we can keep these spaces shaded, covered with litter, or filled in with grass, we can greatly reduce the growth rate and ability of these species to reproduce."
Onsager is intrigued by a technique developed by Llewellyn L. Manske for the Northern Great Plains. Manske is a range scientist at North Dakota Stale University's field station in Dickinson. His "twice over" rotation correlates grazing to the life stages of important native prairie grasses.
Manske starts the first round of cattle grazing when the grasses get their third leaf and stops when they flower. His studies have shown that this approach stimulates extra sprout, or tiller, growth. The additional forage feeds the cows during a second round of grazing and fills in the open spaces during critical grasshopper breeding times.
"Instead of the ecosystem performing like a couch potato, we can get it to perform like a well-trained athlete," Manske says.
Grazing allotments managed according to Manske's methods contained 66 to 75 percent fewer of the migratory grasshopper (Melanoplus sanguinipes) than surrounding rangeland, according to Onsager's measurements. This species is one of the most destructive and tenacious grasshopper pests in the United States.
Computers Can Also Help
All grasshopper IPM strategies rely on understanding grasshopper biology and accurately predicting outbreaks.
ARS entomologist James R. Fisher is creating computer models to predict hatching of the migratory grasshopper and other economically important species.
 Entomologist Jim Fisher checks a weather station at the Lane Ranch near Three Forks, Montana. Grasshopper eggs hatch when soil temperature and other conditions are just right. (K7055-6) |
Most grasshopper eggs spend the winter in the soil after a temperature-driven dormancy called diapause, says Fisher. When soil temperature and other conditions are right, the eggs hatch. As grasshoppers mature, they shed their hard exoskeleton five times. Each of these nymphal stages is called an instar.
"To effectively manage grasshoppers, you usually have to treat them by the fourth or fifth instar, before they can cause significant forage damage or lay eggs," Fisher says. "If we can predict when they will hatch, we can plan the best time to apply treatments."
He expects to complete a prototype of his hatch model by next year. Initially the model will calculate the hatch time of at least two key grasshopper species to within a week.
J. Ross Wight and Fred B. Pierson are creating a related program to correlate soil temperature with air temperature. Soil temperature is a better indicator of egg hatch, but air temperature is more commonly monitored. Both Wight and Pierson work at the ARS Northwest Watershed Research Laboratory in Boise, Idaho.
ARS scientists are already using a streamlined hatch and growth model to give APHIS high-tech ways to automate their grasshopper survey and prediction system.
APHIS specialists count grasshoppers and identify species in the western stales each summer. Until now, they have plotted the information on hand-drawn maps. Soon they will simply provide ARS with a diskette containing the survey information, and the computer will prepare the maps.
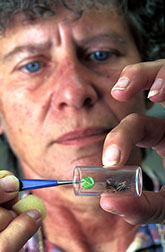 At the ARS Rangeland Insect Laboratory in Bozeman, Montana, microbiologist Elaine Oma places a pathogen-infected leaf in a tube with a test grasshopper. (K7055-8) |
"This automation will increase efficiency, improve uniformity, and help us maintain an electronic record of the data," says John Larsen, who is APHIS' Plant Health Director for Wyoming.
ARS entomologist William P. Kemp, who leads the ARS lab in Bozeman, and computer specialist Tom Kalaris have designed the map-producing system. They combine satellite global positioning system (GPS) technology with powerful geographic information system (CIS) computer software to plot and extrapolate data on grasshopper populations. The hatch model allows them to predict potential trouble spots from this year's grasshopper survey.
"These maps show us where to watch closely for signs of an outbreak," Kemp says.
In a pilot project last year, Kalaris demonstrated that he can provide the maps within a few hours of receiving the data from the field.
"Generally, land managers would get maps within a week," Kalaris says, "but we can help them immediately if there's a crisis."
Scientists hope to add weather patterns, ecosystem types, and species information to the maps, increasing the accuracy of their predictions.
But the most popular computer technology to come from the project will likely continue to be Hopper. This easy-to-use program helps land managers choose the most economical control option for their situation.
Hopper integrates existing biological knowledge with livestock economics and pest control costs. APHIS policy now mandates that Hopper be consulted before the agency launches a control project. Hopper is the work of Kemp, Onsager, economists Melvin Skold and Robert Davis at Colorado State University, and James S. Berry, a former ARS entomologist who now works for APHIS.
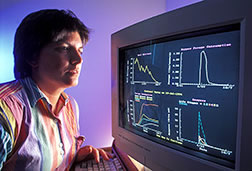 Montana State IPM's Sue Blodgett uses the Hopper computer program. (K7055-9) |
Hopper's most startling advice on how to best manage an outbreak may be to sometimes simply leave the grasshoppers alone.
"In the past,” says APHIS' Gary Cunningham, "grasshoppers were considered an economic threat if there were more than eight per square yard. As a result, in part, of the IPM project, we're starting to look at all the factors that affect grasshopper problems before deciding to implement controls.
"In some situations, Hopper might indicate that it's more cost-effective for ranchers to buy hay for livestock than to pay for a grasshopper treatment," he says.
Though the research project is officially over, the benefits are just starting to be realized. A state-sponsored National Grasshopper Management Board will serve as a future forum for updating the user handbook and advising researchers and policymaker.
"Cyclical grasshopper outbreaks will continue to be a problem in the future," says Cunningham. "Products like the user handbook and Hopper are the legacy that will be usable beyond the project's lifetime."—By Kathryn Barry Stelljes, ARS.
For more information on the grasshopper IPM project, contact USDA-ARS Northern Plains Agricultural Research Lab, Sidney, Mont.; phone 406-433-2020.
How To Get the Handbook and Software USDA Animal and Plant Health Inspection Services Technical Bulletin No. 1809, issued Spring 1996 - Summer 2000 The Grasshopper IPM User Handbook describes the results of grasshopper research by ARS and other project scientists in nontechnical terms. It is available in PDF from the USDA/ARS Northern Plains Agricultural Research Lab in Sidney, Montana. Hopper, version 4.0, runs on IBM-compatible computers. |
Grasshoppers Frozen in Time
Grasshoppers, migrating over mountain ranges as long as 800 years ago, became trapped in snow and ice. Scientists are now chipping these insects from 11,000-foot-elevation glaciers in Wyoming and Montana.
The glaciers have been receding in recent years, exposing insect life as it existed in pre-Columbian limes. As much as 90 percent of some glaciers has melted in the past 85 years.
"We especially want to discover why one species known as the Rocky Mountain locust—which numbered in the billions periodically—mysteriously began to disappear in the late 1800's and became extinct in the early 1900's," says Richard Nunamaker. ARS entomologist in Laramie, Wyoming. He was a volunteer member of a research team headed by Jeffrey Lockwood at the University of Wyoming.
Any additional understanding of grasshopper ecology may help in management of pest insects and conservation of beneficial insects.
Furthermore, state-of-the-art techniques for DNA analysis might help researchers understand the taxonomic relationship between the extinct grasshopper and modern-day species. And the results might reveal the process that led to the locust extinction, perhaps a genetic trail that decreased their environmental vigor.
Nunamaker went on two specimen-gathering trips to the Knife Point Glacier in Wyoming's Shoshone National Forest. He collected frozen butterflies, moths, bees, ants, crickets, and dragonflies, in addition to the grasshoppers. Lockwood studied four other glaciers in Montana.
"The Rocky Mountain locust specimens we collected were far better preserved than any previously obtained from glaciers. We had to gather them quickly, though. Birds found them a delicacy and pulled them out as soon as ice melted. And if we and the birds weren't fast enough, melting water quickly washed them away," adds Nunamaker.—By Dennis Senft, ARS
"IPM Targets Grasshoppers" was published in the January 1996 issue of Agricultural Research magazine.







